Cold Chain
Vaccines are made from micro-organisms similar to the ones that cause disease, or from the toxins that bacteria produce. Therefore, all vaccines are sensitive biological substances that progressively lose their potency (i.e. their ability to give protection against disease). This loss of potency is much faster when the vaccine is exposed to temperatures outside the recommended storage range.
In addition to being temperature-sensitive, several vaccines are also highly sensitive to strong light, and thus need to be kept in the dark as much as possible. They are given some protection by being supplied in vials of dark brown glass to reduce the penetration of light, but this alone will not prevent light damage however and great care must be taken to protect them during use.
Each exposure to extreme temperature or strong light results in some degradation of the vaccine. Furthermore, each exposure to inadequate conditions has a cumulative impact on vaccine potency. Once vaccine potency has been lost, returning the vaccine to correct storage condition cannot restore it. Vaccines do not change their appearance when losing its potency so it is not possible to see whether a vaccine in a vial has lost its potency without a complete laboratory test. Any loss of potency is permanent and irreversible.
The temperature range required for each vaccine is established by the manufacturer. Most of the vaccines require between +2°C and +8°C, but this may vary according the vaccine and the storage times. For example, some vaccines can be stored at-15°C to -25°C in a central storage, up to 6 months, but only up to 1 month at 0°C to +8°C, in a District and Health Centre storage. Oral Polio vaccine may be thawed and frozen again without danger to the vaccine. Some other vaccines like Inactivated Polio, Diphtheria-pertussis-tetanus, Diphtheria-tetanus, Hepatitis B and Tetanus vaccines are seriously damaged by being frozen at temperatures below 0°C. Always refer to the vaccine manufacturer specifications to store the vaccine within the suited temperature ranges during the appropriate timeframes.
The cold chain includes all the equipment and practices used to ensure a constant temperature for a product that is not thermostable (such as vaccines, serums, tests, etc.), from the time it is manufactured until the time it is used. It also includes all the temperature monitoring equipment and routines.
Health workers and logisticians involved in vaccines management are responsible for maintaining the adequate storage and transport conditions: while vaccines are stored in the vaccine stores at the province and county levels, or while they are being transported to township and villages, and while they are being used during immunisation sessions or rounds.
All relevant staff must be trained to both use and manage the cold chain equipment and to regularly monitor the temperature. This includes having appropriate and efficient logistics mechanisms to manage shipping, fuel, spare parts etc.
Common Terms in Cold Chain
|
Cold Box |
Insulated containers that can be lined with coolant packs to keep vaccines and diluents cold during transportation and/or short period storage. Cold boxes are used to collect and transport vaccine supplies from one fixed vaccine store to another, and from vaccine stores to health facilities. They are sometimes also used to temporarily store vaccines when the refrigerator is out of order or being defrosted. |
|---|---|
|
Cold Chain |
Equipment and practices used to ensure a constant temperature for a product that is not thermostable (such as vaccines, serums, tests, etc.), from the time it is manufactured until the time it is used. It also includes all the temperature monitoring equipment and routines. |
|
Cold Life |
The number of hours the temperature inside a passive cold chain container stays below +8º C. This depends on the ambient temperature, the number of times the box is opened and for how long, the number and temperature of the ice packs used, but also on the quality of the box, how well it closes and insulates. Cold life tests are performed at +43º C. Do not confuse “Cold life” with “Cool life”. |
|
Cool Life |
The number of hours the temperature inside a passive cold chain container stays below +20º C. |
|
Coolant Pack |
Also referred as “ice packs”, are flat, square plastic bottles that are filled with water and cooled. They are used to keep vaccines cool inside the vaccine carrier or cold box. |
|
Decommission |
The process of planned removal of equipment from an active status and its storage in a secure and safe place until disposal. |
|
Disposable Insulated Carton Box |
Passive cold chain portable container used by producers to ship their vaccines around the world. Generally, they consist of a polystyrene box inserted in a cardboard box for transport of large quantities of vaccines in favourable circumstances (e.g. in an airplane). They have a limited cold life (often with a maximum of 4 days). |
|
Lot Release |
The process of national regulatory authority evaluation of an individual lot of a licensed vaccine before giving approval for its release on to the market. |
|
Summary Protocol |
A document summarizing all manufacturing steps and test results for a lot of vaccine, which is certified and signed by the responsible person of the manufacturing company. Also called “lot summary protocol” |
|
Vaccine Carrier |
Small cold boxes, portable by one single person, used to keep the vaccine cold for short transport, or to store vaccines temporary just before vaccine administration. There are many types. |
|
Expanded Programme on Immunization (EPI) |
Global program initiated by WHO with the objectives to ensure immunization of all children against certain diseases (such as measles, rubella and tetanus) and to eradicate poliomyelitis, and to extend all new vaccine and preventative health interventions to children in all districts in the world. |
|
WHO PQS |
WHO audited and pre-qualified medical equipment based on Performance, Quality and Safety (PQS) requirements. The list of validated equipment is accessible online and used by several agencies as reference for procurement. |
|
Ready-to-use Vaccine |
Vaccines that come as a liquid and are ready to use in the person. |
|
Reconstituted Vaccines |
The vaccines that come in a lyophilized (or freeze-dried) state and need to be reconstituted at the vaccination site. The latter come in two vials: one for the lyophilized vaccine, the other containing the diluent (saline solution). |
|
Solar Direct-Drive (SDD) |
Refrigeration technology for solar powered devices that avoids the batteries for energy storage. |
|
Vaccine Preparation |
The process of mixing freeze-dried vaccine with the diluent. Consider that vaccine produced by one manufacturer must never be used with diluent produced by another. |
|
Sensing/Monitoring Equipment |
Specialized instruments that remotely monitor and log data on cold chain facilities, including ongoing inside/outside temperatures, and temperature events. |
Cold Chain Equipment
There are two main ways to preserve temperature in the ranges that the vaccines require:
- Keeping the vaccines in a container able to continuously produce cold by itself (i.e. an electric fridge).
- Keeping the vaccines in a container together with a cold material able to emanate cold for a certain period of time (i.e. a box loaded with ice).
The first method is called active cold chain, as the container “actively” produces the cold required. Such devices are commonly referred as refrigeration units. It includes: refrigerators, freezers, cold rooms and air conditioners. It is mostly used for storage.
The second method is called passive cold chain, as the container is passive, and only retains cold from the stored item itself. Such devices are commonly referred as isothermal boxes or insulated shipping containers or passive containers. It includes: cold boxes, vaccine carriers and insulated boxes. It is mostly use for transport.
Active cold chain requires regular energy supply, while passive cold chain requires continuous cold supply, normally water ice, carbon dioxide ice (also known as “dry ice”) or refrigerated or frozen Gel packs.
Active Cold Chain
Active cold chain devices use mechanical or electric systems, powered by an energy source, combined with thermostatic control to maintain desired temperatures.
The main technologies used to produce cold are: compression, absorption, solar battery powered and solar direct-drive.
Compression Refrigerators
Also known as “Mains powered refrigerators”, these are the models most commonly used. They run solely on electricity. These models use little energy, require little maintenance, produce significant amounts of cold quickly and are easy to repair. They are equipped with a thermostat for setting the desired temperature.
Vaccine storage refrigerators are designed to operate in different climatic conditions; some models require as little as eight hours of energy per day. They are built with double wall and an internal ice lining surrounding the vaccine storage area. The frozen icepacks maintain the temperature below +8°C during lack of external power or electricity supply failures. They are known as Ice-Lined Refrigerators (ILRs).
Compression type refrigerators are loaded with a coolant fluid agent which in the form of gas is pumped by a compressor to the condenser where it forms as a liquid. This liquid later vaporises in the evaporator, capturing heat and therefore cooling the surrounding air. The gas returns to the compressor to begin the cycle again, as long as the thermostat keeps the circuit closed and the compressor running.
There are four different types of compression refrigerators and freezers: (1) Refrigerator only, (2) Freezer only, (3) Refrigerator and Freezer (with different compartments), and (4) Refrigerator or Freezer (the entire unit is used either as a refrigerator or as a freezer).
Absorption Refrigerators
These types of devices draw energy from kerosene or gas (butane or propane) normally combined with an alternative electrical connection. The coolant agent used in these devices is a solution of water, ammonia, hydrogen with a small quantity of anti-corrosion. The cooling circuit is closed; therefore, it is not possible to fill it with or repair it if there is a leak.
They are suitable for situations where electricity is not available or reliable, but running costs are expensive due to its continuous fuel consumption and it can be difficult to control the temperature within the recommended parameters. Moreover, absorption refrigerators are less energy efficient, produce less cold, are slower, environmentally unfriendly and contribute to greenhouse gasses.
Because of all the above-mentioned reasons, some agencies no longer recommend the procurement of absorption type refrigerators, preferring solar powered systems. However, this type of devices is still used in some remote regions.
Solar Battery-Powered Models
Battery-powered solar refrigerators were introduced as an alternative to the absorption type refrigerators. They are a solution to the challenges of storing vaccines in locations without reliable electricity or with electricity problems.
In the battery-powered solar refrigerators, the power flows from the solar panels to the battery though a charge controller, which also releases the power either from the panels or from the batteries to the refrigerator. In the absence of sunlight during cloudy days or at night, the refrigerator relies in the energy stored in the lead batteries. In these devices, the power is used to run a DC compressor, pushing the refrigerant through the cooling system, following a similar cycle as in any other compression type refrigerator.
However, experience gained over the time has shown that this technology is more expensive than absorption and grid-powered options. Furthermore, the degree of reliability of solar power decreases as lead-acid batteries require maintenance, are often used for other purposes and must be replaced about every three years. In addition, the batteries contain toxic materials that are difficult to dispose of safely.
Solar Direct-Drive Models
Solar direct-drive (SDD) models eliminate the dependency on the batteries used to power solar refrigerators. The energy is directly supplied by the solar panels: when sufficient light is captured, a DC compressor pushes the refrigerant through the cooling system to form ice in a compartment separate from the vaccine storage unit. This ice bank serves to store thermal energy rather than chemical energy, keeping the fridge cold even in the absence of sun.
There are two categories of SDD refrigerators – those that are entirely battery-free and those that use a smaller, ancillary battery to assist fans and controls. Ancillary batteries used in SDD refrigerators require eventual replacement and project planning must include this cost and consideration. However, the ancillary batteries are much smaller and less costly than those used to power compressor motors in first-generation battery-powered systems.
Passive Cold Chain
Passive Cold chain devices do not produce cold but can maintain temperature for a limited time. Passive solutions are mainly used for keeping vaccines cold during transportation. The technology is fairly simple and requires low level of skills: pre-cooled packs (normally with frozen water, carbon dioxide or gel) are put into an insulated box packed together with the vaccines.
There are two main type of devices - reusable containers (cold boxes and vaccine carriers) and disposable boxes.
Cold Boxes - Insulated reusable containers that loaded with coolant packs are used to transport vaccine supplies between different vaccine stores or to health facilities. They are also used to temporarily store vaccines when the refrigerator is out of order or being defrosted.
The vaccine storage capacity of cold boxes ranges between 5 and 25 Litres and its cold life can vary from a minimum of 48 hours to a minimum of 96 hours (known respectively as “short range” and “long range” cold boxes).

Vaccine Carriers - Insulated reusable containers that, when lined with coolant packs, keep vaccines (and diluents) cold during transportation from health facilities with refrigeration to vaccination sites where refrigeration and ice are not available. They are smaller than cold boxes and therefore easier to carry by a single health worker travelling on foot or by other means, where the combined journey time and immunisation activity ranges from a few hours to a whole day. The vaccine storage capacity of vaccine carriers are between 0.1 and 5.0 Litres.
Disposable Insulated Boxes - (also known as Insulated shipping containers) Insulated containers, manufactured in carton or moulded foams such as polyurethane, polyethylene or expanded polystyrene (EPS). Some are designed for single use while others are returnable for reuse. They are used for the transport of vaccines over long distances. Normally used for products delivery from the central suppliers to main vaccine stores. Their storage capacity, temperature range, cold life and resistance vary among different solutions: some solutions are suitable for Road transport with hold on times between 36-48 hours while some other solutions are suitable for air transport with hold on times up to 120 hours. One main concern related to disposable insulated carton boxes is its single-use lifespan and its low-cost material composition of EPS and water-based gel packs, rarely recyclable.

Cold Chain Storage for Vaccines
Estimating the Required Storage
The required storage capacity will determine the right cold chain solution. The required storage capacity for a given cold chain will depend on:
- The quantity
- The type of vaccines to be stored
Quantity of Vaccines - will depend on the targeted population to be immunised, the vaccine consumption and the vaccine supply frequency and reliability.
The calculation of vaccine consumption requires a careful analysis of existing public health data, targeted coverage and a consideration of future requirements. It is important to plan not only for current needs, but also for future needs.
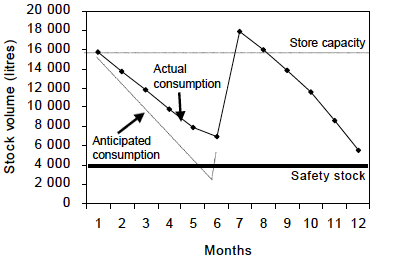
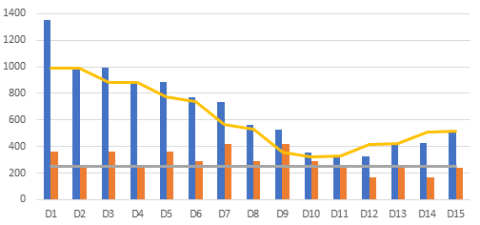
It is advised to add a safety margin in the storage capacity to respond to stock peaks that may overwhelm the cold chain storage capacity (see figure below, taken from WHO Guideline for establishing or improving primary and intermediate vaccine stores). Stock peaks occur when the volume of vaccine actually distributed in the period between any two supply intervals is less than the volume predicted to be distributed during this period. They can also arise if a vaccine delivery arrives earlier than anticipated. Overstocking and under-stocking are also caused by seasonal fluctuations in demand, campaigns, National Immunisation Days and so forth.
In addition to stock peaks, it is recommended to maintain some extra store capacity as back-up to overcome equipment malfunctioning or maintenance (i.e., emptying a refrigerator for defrosting).
Type of Vaccine - of key importance because different vaccines have different presentations. The most common are vials (or ampules), but also, single-dose pre-filled syringes may be stored. Depending on the vaccine, vials can contain different number of doses, normally 1, 10 or 20 doses. Because of these different presentations, the key variables used to calculate the required volume for vaccine storage are the number of doses to be stored and the estimated volume per dose. The estimated volume per dose (or packed vaccine volume) quantifies the space needed to store or transport vaccines and diluents and will depend on the number of doses per vial, the physical size of the vial or ampule (primary package) and the bulkiness of the external packaging (secondary packages).
It is important to consider that some presentations include the diluent in the same packaging as the vaccine. In such cases it is necessary to refrigerate the diluent as well as the vaccine. In all cases, diluents should be refrigerated 24h prior to vaccine preparation. Refrigeration of diluents is normally done in the last step of the vaccine supply chain.
Whenever possible, the packed vaccine volume per dose should be calculated using data from the vaccine manufacturer or supplier. The World Health Organisation (WHO) and the United Nations Children’s Fund (UNICEF) online databases provide access to data on the packed vaccine volume per dose or the dimensions of vaccine and diluent packaging for WHO-pre-qualified vaccines.
To calculate required vaccine storage capacity, it is recommended to use the WHO guidance document for vaccine volume calculation: How to calculate vaccine volumes and cold chain capacity requirements.
Measuring the length, width and height of the vaccine packaging and dividing the resulting volume by the total number of vaccine doses contained in that packaging, may be an alternative method for vaccine volume calculation.
Evaluation of Existing Cold Chains
Cold chain encompasses the infrastructure, the equipment, the people and the management processes and its implementation. The following criteria (adapted from WHO Effective Vaccine Store Management initiative) are universal conditions that can be used during any cold chain assessment.
Assessing the Management Processes
Assess the availability of a cold chain management policy or standard operating procedures. It should be available and applied. Cold chain management policy or standard operating procedures should include clear information on:
- Designated staff members responsible for the cold chain management, including the decision makers accountable for ensuring the required resources.
- Vaccine stock requirements specific to the workplace, both in terms of volume and temperature ranges.
- The “safety stock level” and the “maximum stock level” for each vaccine. Stock levels should be maintained between this range.
- Vaccine ordering and stock management processes, including:
- Stock records.
- Process to order, receive and dispatch vaccines, including the equipment required in all deliveries, such as Freeze indicators.
- Standardised recording and reporting of all stock transactions.
- Process for managing damaged vaccines and respective process for quarantine, disposal or reverse logistics.
- general warehousing practices like periodic physical inventories.
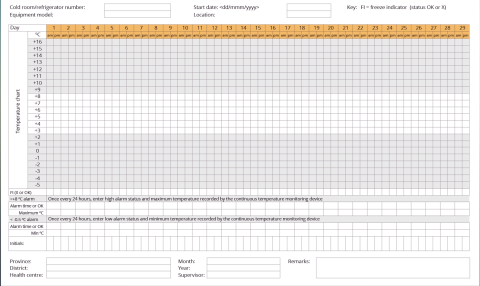
- Temperature monitoring process: required equipment, templates, schedule and reporting processes.
- Operation and maintenance plan, including a specific schedule for all the cold chain equipment. It should include a designated person or service provider responsible for the servicing of the power sources and cooling equipment.
- Actions if the temperature recordings are outside the +2°C to +8°C range.
- Emergency plans and equipment for use in the event of refrigerator failure and/or power outage, including a nominated back –up provider.
- The processes to ensure sufficient funds to cover the required equipment and consumables. A replacement plan for cold chain equipment reaching the end of its lifespan should be considered.
Assessing Cold Chain Equipment
The assessment of the cold chain equipment should include both active and passive cold chain devices as well as other cold chain material such as coolant packs and temperature monitoring equipment. For the existing cold chain equipment, assess:
Capacity - The capacity of cold storage should be sufficient to meet the demand. The store should be able to accommodate peak stock levels for all the vaccines in the program, including campaign vaccines.
Load - The vaccines should be correctly arranged inside the refrigerators, letting enough space for the cool air to flow. Each device containing vaccines should be equipped with (at least) one thermometer or data logger. Temperature monitoring sheets should be attached to the device and records up to date.
Temperature performance - All vaccines should be stored within recommended temperature ranges. Continuous temperature records should be available, and demonstrate that vaccine has been stored correctly. Devices used for temperature recording should have an accuracy of ± 0.5°C.
There shouldn’t be evidence of freezing in the vaccine compartment (noting the means of verification: electronic monitoring data, manual temperature data, shake test). Presence of frozen vaccine vials should be checked as well as the presence of water droplets or wetness on walls of the vaccine compartment or on vaccine vials or boxes
Condition and robustness - Assess if the equipment broke or malfunctioned in the near past and how often this happened. Also, if repairs were performed, indicate the type of repair and if spare parts were used. It is important to consider if the malfunctioning resulted in the inability to use the equipment.
The age and past handling of the equipment could be a vulnerability factor: assess its first “use date” and past history. Be aware that refrigerators have a life duration of about 10 years.
Assessing Infrastructure
The infrastructure should enable the cold store to function effectively. This includes the adequateness of the store building (the location and the construction standard) and the basic utilities, particularly the power supply feeding the active cold chain.
All infrastructure should be of a satisfactory standard and correctly maintained through planned preventive maintenance. Emergency repairs should be exceptional and conducted in a timely manner. There should exist reports on maintenance and repairs. Adequate supplies of spare parts, consumables and fuel should be available. If relying in an emergency generator, it should be also well serviced and operational.
Assessing Human Resources
The assessment of the human resources involved in the cold chain management should include (1) the responsibilities, (2) the correct staffing and (3) the knowledge and capacities.
The responsibilities and tasks should be clearly specified for each person with a role to play in the cold chain management. To assess it, verifying the availability and accessibility to job descriptions is the straight manner. Responsibilities related to cold chain management should be described for all management levels and for every step of the chain: from the personnel monitoring temperatures to decision makers and budget holders.
There should be enough Human resources to operate the store effectively. Even in the smaller vaccine store sites, two or more staff should be appointed to ensure the coverage during unexpected events. A work-plan should guarantee the coverage throughout the whole year.
All the pertinent personnel should be trained in the management of the cold chain. Records of assistance to briefing or training sessions could demonstrate these. In addition, the personnel should have access to the guidance policy or the standard operational procedures.
Suitable Technology and Equipment for Storage
Operational Needs
The operational needs are basically determined by the type of service aimed to be covered and the capacity of storage required. Because of the level of investment and the strategic importance of the cold chain, it is important to apply a long-term vision and plan the needs for the next 5-10 years.
It is important to determine the function of the storage facility within the whole vaccine supply chain and to decide whether or not the site will need to produce coolant packs for outreach, mass vaccination campaigns or recurrent vaccines delivery to other sites. This is mainly because coolant packs should not be stored in the same compartment as vaccines. Therefore, in case coolant packs are to be regularly managed, facilities should use either dual compartment devices, or two separate devices – one for storing vaccines and one for storing coolant packs. In this last scenario, small front-opening refrigerators or refrigerator/freezer combinations are best used at health facilities, where easy access to vaccine and a separate freezing compartment for ice packs are needed. The chosen refrigeration solution should have the capacity to store the required number of vaccines and produce the required amount of coolant packs.
Check the information above about Estimating the required storage capacity to determine the needs and then, the right device size for a facility.
Keep in mind that the capacity of refrigerators ranges approximately between 30L and 200L of net vaccine storage volume. For storage facilities with required capacity exceeding a considerable number of refrigerators, a cold room could be less flexible but a more efficient solution. Walk-in cold rooms (WIC) and walk-in freezer rooms (WIF) are refrigerated enclosures accessible via at least one door and large enough for a person to walk into, housed within existing buildings. Consider that WICs and WIFs are compression type refrigerators and require reliable power supply.
WICs and WIFs are an important storage point in the temperature-controlled supply chain and usually used at the central or national level or near shipping ports used for import/export of vaccines. For comprehensive information on how to choose the correct Walk-in cold rooms (WIC) and walk-in freezer rooms (WIF), refer to UNICEF Procurement Guidelines for Walk-In Cold Rooms And Freezer Rooms.
Top-opening refrigerators and freezers (also known as chest refrigerators and freezers) are the first choice for bulk vaccine storage in places where cold rooms or freezer rooms are not justified.
Context and Available Infrastructure
The context of use and the available infrastructure will influence the access to different power sources, the possibility to use solar devices and the available space and conditions to locate the cold chain equipment.
Accessible Power Sources
It is of key importance to ensure the reliability of the power source for the active cold chain: mains, solar, kerosene or butane. Having access to reliable electricity will back the use of electricity-powered devices – such as ILRs and on-grid freezers – since they have a lower Total Cost of Ownership than solar or passive devices for the same amount of storage.
Off-grid facilities should use devices that can generate their own power (such as SDDs), devices that can keep vaccines cold for long periods of time without power (such as long-term passive devices) or absorption type refrigerators (such as butane or kerosene refrigerators). These devices often cost much more to purchase than on-grid devices, and their operational and maintenance costs tend to be higher than for the electricity-powered devices. Therefore, it is of key importance to ensure ongoing funding in place for the lifespan of the equipment.
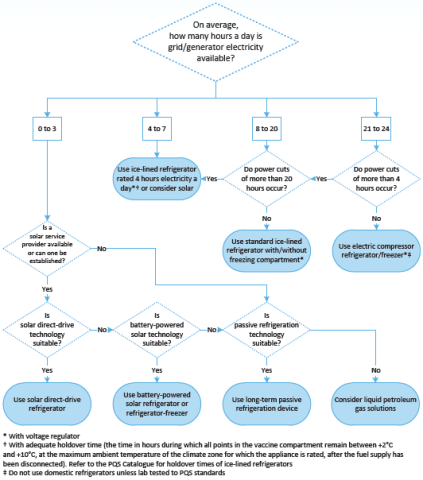
The choice of devices should correspond to the number of hours of electricity that a facility can access per day, and the length of electricity outages it experiences. WHO and UNICEF recommend that all primary vaccine stores should be fitted with a generator with automatic start up, regardless of the reliability of the mains power supply.
Solar devices are suitable for those facilities with sufficient solar energy available at installation locations: basically, strong enough sunlight all year round and clear surroundings without buildings or trees. Be aware that mountainous areas and coastal regions may have micro-climates with prolonged cloud cover. In these cases, the choice of solar technology will be limited and its implementation will require careful design to ensure adequate performance. In addition, it is advised to have a solar service provider able to deliver all necessary services, including site assessments, equipment installation, training, corrective maintenance, and repair.
The following figure, taken from WHO, Introducing Solar-Powered Vaccine Refrigerator And Freezer Systems, A Guide For Managers In National Immunisation Programmes, provides a decision tree to guide in the selection of the most appropriate energy source for vaccine refrigeration:
Room Available
Cold Rooms are bulky items and require considering if the model ordered will fit into the allocated room (including height), allowing for enough space for access and ventilation. Some are prefabricated, allowing to be assembled in any pattern and to any size, with quick and easy installation which can be applied to any context. For further instructions on the installation and pre-operation of Cold Rooms and Freezer Rooms, follow UNICEF Procurement Guidelines for Walk-In Cold Rooms and Freezer Rooms,
Refrigerators and freezers are available in several shapes and sizes. Top-opening refrigerators and freezers, although are the first choice for bulk vaccine storage, occupy more floor space per litre of vaccine than front-opening models. Front-opening refrigerators or refrigerator/freezer combinations are easier to accommodate in reduced spaces and offer an easier access to vaccine.
Operating Temperature Range
It is important to take into account, as part of the environment criteria, the ambient operating temperature range where the refrigerator or freezer performs. This information should be provided by the manufacturer. Though a standard is a range between +5°C and +43°C, some models have a maximum ambient operating temperature of +32°C.
Support and Standardisation
As a general rule, when possible and if reviews show that the cold chain is well managed and that temperature monitoring procedures are reliable, select cold chain equipment similar in technology to the one already in place. This has obvious operational advantages.
It is important for the sustainability of the storage facilities to have access to professional in-country installation and maintenance support, including availability of spare parts. In this sense, the device's warranty and after-sales support from the supplier are fundamental.
Environmental Considerations
Environmental criteria should also be considered when choosing the cold chain equipment. Compression type refrigerators are loaded with a coolant fluid agent (refrigerant). Manufacturers select refrigerants to suit the specified operating temperatures. The type of refrigerant used has evolved together with the growing environmental concerns. Until recently, the use of chlorofluorocarbons (CFCs) was widespread, but in 1987 the Montreal Protocol restricted its use due to its effect on the ozone layer. CFCs were replaced by hydrofluorocarbons (HFCs), like R134a, a popular refrigerant currently in use but still having a high Global Warming Potential (GWP). There is an expected cut-down in the use of HFCs and progressively new production of cooling instruments will probably be limited to Hydrocarbon (HC) refrigerants. HC refrigerants, like R600 or R600a, known as green or natural gases, have a GWP value roughly on the same level as CO2 but are extremely flammable.
Total Cost of Ownership (TCO)
Any vaccine storage site will require a considerable level of investment for its acquisition, installation, operation, maintenance, renewal and decommissioning. Understanding the costs of purchasing and maintaining cold chain equipment over time is basic for the planning and the sustainability of the vaccine storage facilities. To make the proper decision in the selection of equipment, the Total Cost of Ownership for Cold Chain Equipment must be considered.
Domestic appliances, though cheaper and locally available, are considered unsuitable for vaccine storage, particularly in hot climates. WHO recommendations on this matter are as follows:
- Standard domestic refrigerators should only be used for vaccine at the peripheral level, and then only if water bottles are used to improve temperature stability. This is especially true in hot climates. Domestic refrigerators are unsuitable for vaccine storage because they are not designed to maintain the temperature range required and they warm up quickly when the electricity fails.
- Domestic chest freezers should not be used to store vaccines but may be suitable for freezing ice packs.
Other technical reasons to avoid using domestic refrigerators to store thermo-sensitive pharmaceutical products are: its lighter insulation and imprecise regulation of the temperature, the heterogeneity of the temperatures at different areas inside the container and the variations in temperatures in case of automatic defrosting.
The concept of total cost of ownership (TCO) refers to all costs associated with owning and operating a unit of equipment over its useful life expectancy. It helps to evaluate a purchasing decision based on the comprehensive costs of owning and operating a piece of equipment over its useful life or a set period of time. TCO is calculated adding the capital costs and the operating expenses.
Capital costs are one-time costs incurred at the time of purchase. It includes: the costs of the equipment, recommended spare parts, in-country transport, installation kit, and installation labour.
Operating expenses are the recurring costs over the useful life of the equipment. This includes cost of energy (electricity, gas, kerosene), maintenance, repairs and decommissioning plus the costs of operation and training of staff.
Taking in consideration that devices that can generate their own power such as SDDs often cost much more to purchase than on-grid devices and that absorption type refrigerators tend to have higher operation costs, a first step in the process is comparing the costs of the different technologies suitable for the site.
Once a decision on the technology has been taken, a comparison of models will be pertinent. Compare the unit price, useful life, frequency of maintenance required and technical individual device characteristics such as:
- Holdover time for ILRs based on a facility’s power reliability.
- Autonomy time for SDD devices based on regional climate factors.
- Freezer capacity for ice pack production.
- Ease of use, including:
- Readability of control panels and displays by a standing health worker
- Use of internal storage racks, boxes or drawers to help organise vaccines and separate other medicines that are stored in the device.
- Voltage stabiliser integration.
To evaluate the cold chain equipment options from a cost perspective, it is advised to use the “PATH Total Cost of Ownership tool”. The tool allows users to explore the capital and operating costs associated across various cold chain equipment technology categories, as well as to compare costs for specific models within a technology category or across multiple technologies.
For further information on how to choose the correct cold chain equipment, please refer to GAVI’s Cold Chain Equipment Technology Guide.
Installation, Loading and Maintenance
Installation
Installation of cold chain equipment must be done in the adequate room. The room must be accessible for vaccine reception and delivery, large enough, in good building conditions (roof, ceilings, floors, electrical services, etc.) and secure. Useful information can be found in WHO’s Guideline for establishing or improving primary and intermediate vaccine stores and WHO-UNICEF’s Effective Vaccine Store Management Initiative.
Remember that cold chain is not only about refrigeration equipment: storage space for diluents, packaging materials, cold boxes and icepacks should be also considered. A proper planning of the storage room and all needed pre-operation must be completed before installing any active cold chain equipment.
Manuals shall be provided by the manufacturer with each equipment with clear descriptions on procedures for installation, operation, diagnostic and maintenance. Nevertheless, general recommendations for a proper installation of cold chain equipment include:
- Place it out of the sun, away from any source of heat (stove, radiator) and protected from dust.
- The room must be at least 20 m3 of volume, be well aerated and cool if possible; always respect the ambient operating temperature range indicated by the manufacturer.
- Make sure there is clearance between the unit and a wall, partition or other equipment in order to allow air to circulate and facilitate maintenance; the clearance distance should be of at least 25 cm for compression type refrigerators and 40 cm for absorption type refrigerators.
- The refrigerator/freezer should be placed on blocks or pallets to avoid direct contact with the ground, protect against humidity and increase heat evacuation.
- Install it horizontally to ensure good circulation of the cooling fluid.
For equipment powered by electricity, it is essential that it is installed according to the national standards of electrical equipment installation. Devices not having an integrated voltage stabiliser, must be protected relying on standalone voltage stabiliser. This is critical wherever voltage fluctuations exceed ±15% of rated voltage (or the refrigeration equipment manufacturer’s voltage tolerance, whichever is lower).
After the setup of a compression type refrigerator, it is recommended to wait 24 hours before starting it. This is to allow oil lubrication of the compressor, which could have left in the pipes during transport, to go down in the compressor. In the event of starting without waiting, the lack of oil could deteriorate the compressor.
Absorption type refrigerators must be perfectly vertical. To check and to adjust it, a plumb line and a spirit level are located inside the fridge. No waiting period is necessary before using an absorption refrigerator. It can be started immediately.
When possible, and especially if not having in-house capacity, it is advised to outsource the installation of the cold chain equipment. This is especially critical when acquiring SDD equipment or walk-in cold/freezer rooms. It is recommended that full/final cost of the payment for cold store install costs be withheld until a full commissioning test has been completed satisfactorily. Typical commissioning tests should include:
- Cool-down time: Start the refrigeration unit when the room is empty and the same temperature exists inside and outside the room. Keep the cold room door closed during the test. Record the time needed for the internal temperature to drop below +8°C. Run the test for at least 48 hours.
- Running test: Record the number of hours that the compressor runs with the door closed and the room empty. Monitor the internal and external temperatures, the evaporator and condenser temperatures, and the pressures of the system. Measure the maximum temperature difference in the cold room and record the locations of any warm and cold spots.
- Temperature-rise test: Cut off the electricity supply to the room and measure the period required for the internal temperature to rise to 5°C above the normal operating temperature.
- Control and monitoring equipment tests: Test the operation of the automatic duty-sharing, temperature control and temperature-monitoring and alarm equipment. If computerised temperature monitoring is used, load, configure and test the software.
- Stand-by generator operation test: Check the power output of the stand-by generator and the operation of the automatic mains failure control system. Run the generator continuously for 48 hours under load.
For detailed installation instructions for refrigerators and freezers, check EVM Model Standard Operating Procedures, Consolidated version, with user guide, from the Effective Vaccine Management Initiative.
Loading
Basic recommendations for loading the refrigerator units with vaccines, include:
- Do not store unauthorised products (like food or drinks) in refrigerators used for thermo-sensitive products storage. Overload and continuous opening reduce the performance of the equipment and affect negatively temperature stability.
- Keep all vaccine stacked on shelves or baskets, not on the floor.
- Do not append the boxes together or in contact with the walls: leave a space between them to allow cold air to circulate.
- Be careful, not to store freeze-sensitive products in the coldest part of the fridge (in contact with the evaporator): in the bottom for horizontal/top door fridge and on the top for vertical/front door fridges.
- Do not store any vaccine in the doors of vertical/front open refrigerators.
- Keep all vaccines in its inner cardboard box. There should be no loose vials in the store.
- Products should be quickly identifiable in order to reduce the time with the door open. Names and expiry dates must be located on the front and be readable as soon you open the fridge.
- Store the vaccine systematically and keep together the contents of each batch.
- To facilitate handling and reduce the time opening the door, apply a positioning rule. For example, the rule: expiration date closest to the left of the shelf and the farthest to the right. Products with expiry dates closest leave first, then date more distant.
- If there is enough place, the diluents may be stored in the refrigerator also. Remember that vaccine produced by one manufacturer must never be used with diluent produced by another.
- Each refrigerator must be equipped with a thermometer and other mandatory indicators.
For further details on arrangement of vaccines with specific rules for using front or top-opening refrigerators, read WHO’s Immunisation in Practice: A practical guide for health staff.
Maintenance
Cold chain equipment needs periodic maintenance and repairs. Maintenance should be planned from the moment of installation by defining a regular schedule of basic tasks to be implemented by on-site workers.
These regular tasks should include:
- Temperature control and monitoring: This will allow the review of the system performance. Check and record the temperature twice a day. A convenient routine is to do so, first thing in the morning when opening the facility, and last thing in the evening before closing it. Analyse the trend of recorded temperatures and report and investigate any abnormality.
- Visual inspection inside: Weekly check if excessive ice has accumulated in the interior and the evaporator plate. Ice reduces device performance, requiring more electricity, gas, kerosene or solar power. Whenever the ice on the inside lining is thicker than 5mm or at least once per month, defrost the unit and clean and dry the interior of the refrigerator and/or freezer. Regularly check for signs of damage, including corrosion and deformation of the door or lid seal. Carry out repairs as necessary. A door not closing properly or being open too often are the main causes of excessive formation of ice.
- Visual inspection outside: monthly clean and wipe the dust accumulating on the back of the refrigerator and/or freezer (condenser and cooling unit). Excessive dust may reduce device performance. Do not allow rubbish and packaging to accumulate in the vaccine storage area. It is essential to maintain free air movement around the condensing units. Regularly check for signs of rust and carry out repairs as necessary.
- Visual inspection of the source of energy: Maintain the source of energy (mains, voltage stabiliser, batteries, solar panel, etc.) according to the instructions of the manufacturer.
For detailed maintenance instructions for the different type of refrigerators and freezers, including defrosting procedure, check EVM Model Standard Operating Procedures (Consolidated version, with user guide), from the Effective Vaccine Management Initiative.
Basic repairs, such as replacing fuses or repairing rusty surfaces can be performed by on-site workers. To enable this, training of staff is essential. On-site training can be provided by the installation service provider at the time of equipment installation or during a follow-up visit. Other more complex repairs, such those affecting the thermostat, the controllers or the cooling unit, will require a local, regional or central workshop technician. An important element of the maintenance plan is to identify suitable technicians at one or several of these levels able to perform scheduled and emergency repairs.
In any case (regular maintenance such a defrosting or in a breakdown), it is imperative to have the means to relocate the vaccines in another unit during the intervention. The relocation can be done using passive means such cool boxes. Consequently, accurate inventories of the equipment and the basic spare parts are a corner stone of the cold chain maintenance system.
Any malfunctioning, maintenance or repair performed must be consistently reported. This will allow making the correct decisions when a particular unit is not reliable and reviewing the reasoning behind the selection of particular models or technologies in a given context.
Be aware that some types of temperature monitoring equipment are battery powered. These devices contain a non-replaceable battery with a minimum operating life of 2 years from the date of activation. It is essential to include the replacement of these devices as part of their routine preventive maintenance programme.
Decommissioning and Disposal
Decommissioning and disposal are the last stages in the life cycle of the cold chain equipment. When the equipment become technically obsolete or repair and maintenance costs become higher than the remaining value of the equipment, decommissioning and disposal should be considered.
There are several disposal methods depending on the degree of obsolescence of the cold chain equipment: un-repairable, ineffective for vaccines, costly, surplus due to down-scaling of operations, etc. The most common methods for disposal are: donation, transfer, sale, recycling (spare part harvesting) or destroyed.
The development of a decommissioning and disposal policy and practical guideline & tools, is recommended. It should provide guidance on:
- Expected lifespan of the equipment in order to get the best performance and cost-effectiveness.
- Budgeting decommissioning costs.
- Decision process and administrative steps on assets for decommissioning (considerations, responsibilities and traceability).
- Available disposal channels and decision criteria.
- Human and technical available means (for packaging, freight, transport, etc.).
- Environmental considerations.
Obsolete cold chain equipment can be hazardous to people and the environment; therefore, the applied disposal method must consider at least the following aspects:
- Cold chain equipment could have been used for laboratory purposes, storing potentially infectious substances such as: biological material, blood, and body fluid or excreta. In such cases, decontamination by a trained technician should be performed.
- Compression type refrigerators are loaded with a coolant fluid agent (refrigerant) having a high Global Warming Potential. Refrigerant may be recovered, destroyed, reclaimed for sale, or stored safely to prevent emissions.
- Some refrigerators and freezers may have heavy-metal components that should be safely removed prior to final disposal.
- Plans also need to be in place for the safe disposal of used temperature monitoring devices. Ideally, they should be recycled in accordance with local regulations because they contain valuable materials, some of which may be toxic. Electronic devices marketed in the European Union will generally be marked with a warning symbol indicating that the product should not be sent to a landfill.
- Solar equipment (panels and batteries) should be handled separately with its own assessment and disposal procedure.
For further information on decommissioning and disposal, refer to UNICEF guidance on Decommissioning and Safe Disposal of Cold Chain Equipment.
Cold Chain Transport for Vaccines
Effective and secure transport arrangements should be in place for moving vaccines keeping the correct temperatures. The process can be defined in 5 steps:
- Assessing the shipment conditions.
- Deciding the suitable means for vaccine transportation.
- Preparing the shipment.
- Shipping.
- Reception and Cold chain verification.
When opening new distribution channels or recurrent cold chain breaks are experienced in current distribution, qualifying transport routes is recommended. The process typically involves monitoring worst-case routes in order to ensure that the personnel and the chosen equipment and packaging arrangements are able to maintain acceptable transport temperatures even in such cases. Guidance on how to carry out a study of this kind is given in the WHO document Study protocol for temperature monitoring in the vaccine cold chain.
Assessing Shipping Conditions
Calculating Volumes
To calculate the volume of vaccine to be shipped, it is necessary to know for each vaccine and diluent in the shipment:
- The required storage temperature: 3 ranges of temperature are normally considered for vaccine transportation: -15 to -25°C, +2 to +8°C or ambient;
- The number of doses to be transported;
- The packed volume per dose (cm3/dose). The packed volume includes the vaccine vial, the packet containing the vaccine vial and any intermediate packaging (secondary packaging).
The maximum recommended packed volume per vaccine dose and diluents are:
|
Be aware that the volume obtained from multiplying the packed volume per dose by the number of doses only takes into consideration the primary and the secondary packages: it doesn’t include the cold box packaging. Estimating the final transport volume (including the cold box) is necessary to correctly plan the transport means. For this purpose, a transport box bulking factor can be used. The bulking factor depends on the type of vaccine. WHO Guideline for establishing or improving primary and intermediate vaccine stores, recommends the following transport box bulking factors:
- BCF, OPV, measles, MMR, MR = 6.0
- Other vaccines = 3.0
- Diluent, droppers = 1.5
Evaluating the Journey
To evaluate the journey, some of the criteria to be consider are:
- The transport modes and vehicle types.
- The journey distances and its expected duration.
- The environmental conditions: temperature (day–night and seasonal temperature extremes) and geographical and natural hazards.
There are 3 basic transportation stages in the supply chain of vaccines:
- From the manufacturer to a primary or central store: usually international shipments.
- Between (intermediary) stores: normally between national or district store facilities and down to the health care facility.
- Outreach transportation: final vaccine delivery during regular EPI or to a vaccination site during a mass vaccination campaign.
Aerial or terrestrial modes are preferred for vaccine transportation. Air transport is usually chosen for international or long-distance shipments. Terrestrial is used for transport of vaccine within the same country. Outreach is often done by any land transport mode: car, motorcycle, bicycle. Because of the long duration of the journeys, vaccines are rarely transported through waterborne means.
For further information on how to design a vaccine distribution system, refer to WHO Guideline for establishing or improving primary and intermediate vaccine stores.
Suitable Containers for Vaccine Transportation
There are several alternatives for the shipment of temperature-sensitive cargo:
Refrigerated (Multimodal) Containers
A refrigerated (multimodal) container or reefer, is a shipping container equipped with an integrated refrigeration unit for the transportation of temperature-sensitive cargo. They rely on external electrical power provided from the ship, the quay or the trailer. These type of containers are suitable for large-scale shipments and when the journey requires changing modes of transport (i.e. road-sea-road), normally over long distances.
Refrigerated containers are rarely used for vaccine transport. For long distances or inter-continental movements, vaccines are commonly air-shipped in cold storage containers, which are either actively powered or passively kept cold. Therefore, refrigerated multimodal containers are not advised for transportation of vaccines.
Refrigerated Vehicles
A refrigerated vehicle is a van or truck with a thermally isolated cargo compartment, equipped with a mechanical refrigeration system for road freight transport at specific temperatures.
Such vehicles are utilised for large-scale transport of vaccines from the manufacturer to primary/central stores and in certain context for bulk transport between primary/central stores and secondary stores. Refrigerated vehicles are commonly run by specialised logistics operators. Please review the checklist for contracting refrigerated vehicles in the road transport section of this guide.
Still, the high cost of refrigerated vehicles and their tendency to suffer mechanical breakdowns, have prevented many developing countries from using this transport method for regular deliveries. Additionally, when using a refrigerated vehicle in such contexts, it is recommended cold packing provisions to protect vaccines in case of vehicle breakdown.
Given that some cold boxes, if properly loaded, have enough cold life to cover transportation needs at national level, the use of refrigerated vehicles for bulk transport of vaccines is also discouraged if reliable infrastructure is not accessible.
Portable Passive Vaccine Containers
A portable passive vaccine container is a container that maintains a temperature-controlled environment inside an insulated enclosure, generally without thermostatic regulation, using frozen ice-packs, conditioned ice-packs, cool water-packs or warm water-packs. In this guidance, it includes reusable insulated cold boxes and vaccine carriers as well as single-use insulated cartons. Because of the different models available, its versatility and its cost, they are the most used containers for the transport of vaccines.
There are three main types of portable containers: disposable insulated carton boxes, cold boxes and vaccine carriers.
Disposable insulated carton boxes are used by manufacturers to ship their vaccines around the world. They must conform to certain standards. They have a cold life often with a maximum of 4 days.
Three categories of vaccine packaging are used for international air freighting (listed below in decreasing order of bulk):
|
Class A |
packaging is designed to ensure that the temperature of the vaccine does not rise above +8°C for a minimum exposure of 48 hours at an ambient temperature of 43°C. |
|---|---|
|
Class B |
packaging is designed to ensure that the temperature of the vaccine does not rise above +30°C for a minimum exposure of 48 hours at an ambient temperature of 43°C. It must also prevent the temperature of the vaccine from dropping below +2°C for a minimum of 48 hours at an ambient temperature of -5°C. |
|
Class C |
packaging provides no specific protection against high temperatures. However, it must prevent the temperature of the vaccine from dropping below +2°C for a minimum exposure of 48 hours at an ambient temperature of -5°C. |
Based on its dimensions and handling, insulated vaccine containers can be either (1) an individual insulated shipping carton or (2) a pallet shipper. It is recommended that each insulated carton should weigh less than 50kg to ensure ease of handling during transport as they are frequently loaded and offloaded manually. Pallet shippers have a built in wooden or plastic pallet platform to enable handling and transport by forklift or pallet handling equipment. Pallet shippers will generally accommodate higher volumes of vaccines per unit. It is recommended that external dimensions of pallet shippers for vaccines should not exceed standard ISO pallet sizes (US Pallet 1200mm x 1000mm, or Euro Pallet 1200mm x 800mm 276). Pallet shipper height should not exceed 1600mm.
Due to infrastructure and logistics constraints in some locations, it is advised to assess the logistics capacity downstream. In case of limited logistics capacity, it is preferable to ship vaccines using individual insulated cartons.
|
Cold Boxes |
Reusable containers generally used to transport vaccines from one fixed vaccine store to another, and from vaccine stores to health facilities. They have a vaccine storage capacity between 5.0 and 25.0 litres. There are two types of cold boxes:
|
|---|---|
|
Vaccine Carriers |
Used for transporting vaccines where the combined journey time and immunisation activity ranges from a few hours to a whole day. The vaccine storage capacity of vaccine carriers is between 0.1 and 5.0 litres. |
When choosing means for transport of vaccines, consider the following factors:
- The heat and freeze sensitivity of every vaccine being transported. If available, refer to manufacturer indications for further information on temperature sensitivity of vaccines. In any other case refer to WHO How to use passive containers and coolant-packs.
- The required cold life to keep vaccines at safe temperatures for an entire transport or outreach session. For vaccination outreach sessions the considered time should include travel to and from the vaccination site, allowing the safe management of non-used vaccines.
- The required capacity based on the volume of vaccines to be transported.
When selecting the appropriate container, the time of transport must be considerably less than the cold life of the container. Unexpected events such as vehicle breakdowns, human error or carelessness, often delay the time of transport. When the duration of the journey exceeds the cold life of the container, it is possible to replace the coolant packs if necessary. The back-up coolant packs can be transported in a separated container or swapped in a stop-by storage facility with compatible coolant packs. It is therefore necessary not to compromise on the number of ice packs which may need to be prepared.
Coolant Packs
Once the decision about the type of container is taken, calculate the number of cold boxes required. Subsequently calculate the number of coolant packs and temperature tracking and alert devices required. Each container holds a specific number of coolant packs.
In regular cold chain management, it is recommended that every cold box or vaccine carrier should have at least two sets of coolant packs, allowing one set of the packs to be cooled, while the other set is being used in the cold box or vaccine carrier. Note that one set of coolant packs is normally provided with each procured cold box or vaccine carrier, so that one additional set at least needs to be ordered.
The type of coolant packs must be selected according to the container and the required temperatures. Ideally, they should be compatible with other coolant packs used in the country.
There are several types of coolant packs:
|
Water-Filled Coolant Packs |
The most commonly used, they are available in a solid rectangular plastic container in different sizes. The most common are: 0.3 litres (in two different sizes: 173x120x26mm and 163x90x34mm), 0.4 litres (163x94x34mm) and 0.6 litres (190x120x34mm). They are used to maintain temperatures in reusable cold boxes or vaccine carriers. WHO currently recommends the use of water-filled coolant packs. Drinking water is safe for such use and is generally available; this makes it the most practical substance for filling coolant packs because both water and ice can effectively control the temperature of the vaccine load, when correctly used. |
|---|---|
|
Gel-Packs |
sealed coolant containers pre-filled with a mixture of water and additives. They are available in flexible plastic bag or in a rectangular plastic container. WHO does not recommend using gel-packs because their thermal properties (freezing point of some gel-packs can be significantly below 0°C) and their lower durability. |
|
Phase-Change Material Packs (PCM-packs) |
containers filled with other phase-change materials different from water. They can be designed to change phase at the convenient temperatures range, overcoming the vaccine freezing risk associated with frozen water. However, they are also more expensive and their conditioning process is longer and more complex. |
Vaccine manufacturers ship products by air using coolant-packs of various types and sizes containing various fill materials, including water, gel and PCM. It is a common practice to reuse these coolant packs recovered from international shipping containers. WHO discourages this practice as these packs do not necessarily perform in the same way as the water-packs. In addition, they are not designed for repeated use and may not be dimensionally compatible with most of the passive containers used for the in-country supply chain. The recommendation is that, after vaccine arrival, these packs are removed from the receiving vaccine store and recycled or disposed of according to the vaccine manufacturer’s recommendations and/or national waste management policies.
Preparing Shipments
Conditioning Water-Packs
The temperature of coolant packs must be set according to the temperatures required by the vaccines to be shipped. There are two main possibilities: (1) the vaccines to be shipped in the cold box may be frozen (Measles, Polio, Yellow fever, Meningitis, etc.); (2) the vaccines to be shipped in the cold box will be irreversibly damaged when frozen (DTP, DT, Td, TT, Hep A and Hep B, Hib).
If all vaccines to be shipped in the cold box may be frozen, frozen coolant packs can be directly transferred from the freezer to the cold box.
In the case that vaccines will be damaged when frozen, the coolant packs need to be “conditioned” before being transferred into the cold box. That means bringing its temperature up to 0ºC. The conditioning of coolant packs consists of laying the required number of frozen ice-packs on a table or work surface (preferably not under direct sun light) and waiting until they all reach 0ºC. This may take at least 30 to 45 minutes in hot weather and much longer in cool conditions (from 90 to 120 minutes at +20°C). In order to know when the icepacks are ready to be used, there must be liquid water inside every pack and the ice cores should be able to move freely inside the packs when shaken. To ease the process, place the icepacks in one single layer and separated from each other.
The use of cool water-packs and warm water-packs can be pertinent for some shipments. Warm water-packs are used to protect freeze-sensitive vaccines in countries where temperatures are frequently below 0°C. Warm water-packs are to be prepared at a room temperature between +18°C and a maximum of +24°C. Cool water-packs are to be prepared in a refrigerator at a temperature of no more than +5°.
For further information on the preparation of passive containers with frozen, conditioned, cool, or warm water packs, refer to WHO’s How to use passive containers and coolant-packs.
Packing
Correct packing arrangements are necessary to prevent temperature excursions and vial breakage. In addition, it makes efficient use of the available storage volume.
The first action during packing is to dry any droplets on the coolant packs surface and placing it in a cold box according to the cold box manufacturer specifications: the correct size and number of coolant-packs must be used. The technical sheet for loading the cold box is often available inside each box.
Place the vaccines inside the cold box, putting cardboard between thermo-sensitive products and icepacks to prevent them touching. Make sure that any remaining space is filled with packing material to avoid damage during onward transport.
When packing vaccines without secondary package/carton box (common practice when using vaccine carriers), put the vaccines and diluents in a plastic bag in the middle of the cold box or carrier to protect them from damage due to condensation.
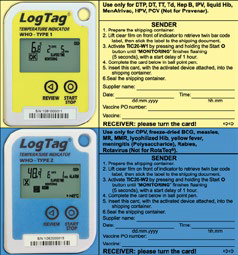
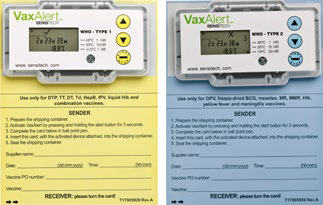
Place the required temperature monitoring devices in the box or carrier. In case of using electronic monitoring devices, do not forget to activate it. Do not allow monitoring devices to come into contact with coolant packs. If using a thermometer in the container, place it in a visible and easily accessible place to avoid long content handling during temperature checks.
When required, put the top layer of coolant packs and close the container.
Labelling Containers
The labelling of containers carrying vaccines must indicate warnings about the time and the temperature sensitiveness of the shipment.
Specific requirements exist for the labelling of international/air shipments. Therefore, a distinction must be made between international/air and domestic shipping.
International/Air Shipments
For international/air shipments, a label must be affixed to the front surface of each package indicating type of vaccine, name of manufacturer, presentation, batch number, date of manufacture, date of expiry, quantity, and storage conditions. The manufacture date and expiry date on all labels should be written in full, not in a coded form (i.e. June 2017, not 06.17). In addition, required temperature conditions for transportation must be clearly visible on the outer carton, indicating clearly where recommended transportation temperatures differ from recommended storage temperatures.
A “Vaccine Rush” Label must be affixed to each face of the vaccine package
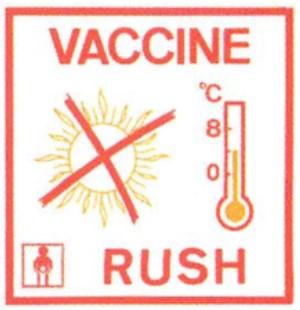
A “Do Not Freeze” label must be affixed to those packages (in each face) containing freeze-sensitive vaccines, droppers or diluents.

An IATA Time and Temperature Sensitive Label (mandatory from 2012). The lower half of the label must never be left blank and must indicate the external transportation temperature range of the shipment - this can be hand written or printed onto the label.

Labels must be written in a language appropriate to the country of destination.
Shipping documents should be included in the box labelled “Number 1”, and this box should be clearly labelled with the words "Containing vaccine shipping documents".
Domestic/Road Shipments
There is no specific international regulation for labelling vaccine shipments transported by road. Nevertheless, becoming knowledgeable in the laws of all of the countries in your distribution channel can help to avoid administrative burden and delivery delays.
In any case it is recommended that shipper and consignee agree on a basic standard operational procedure to pack, label and receive vaccine shipments. Among other topics, the procedure should establish the warning signs about time and temperature sensitiveness of the vaccine parcels. As a minimum standard, it is recommended that a “Vaccine Rush” Label should be affixed to each parcel carrying vaccines.
Shipment Documentation
Having the proper documentation in time is critical for any vaccine shipment as any deficiency may delay it exposing the vaccines to inconvenient temperature conditions, especially through cross-border supply chains. The shipper must provide the cargo details with sufficient time in advance to allow the consignee to prepare for the reception. In addition to the commonly accepted standard set of shipping documents and documents associated with importation, the documents and information should include:
- Date and time for place of departure, transit (if applicable), and arrival.
- Type of vaccine, total number of primary containers/vials and number of doses per primary container/vials.
- Lot Release Certificate issued by the national regulatory authority (NRA) of the country of manufacture for each lot of vaccine supplied, together with the Certificate of Pharmaceutical Product (also by the NRA).
- Lot Summary Protocol of production and quality control.
The following original documents should accompany the consignment when it is shipped:
- Signed and/or stamped supplier’s invoice.
- Packing List.
- Lot Release Certificate issued (signed and/or stamped) by the national regulatory authority of the country of manufacture for each lot of vaccine supplied.
- Lot summary protocol.
One set of the original documents above must also be placed inside the parcel numbered “1”. This particular parcel should be clearly labelled with the words "Containing vaccine shipping documentation".
A list of contact points for national regulatory authorities in countries producing vaccines pre-qualified for purchase by United Nations agencies can be found in the Annex 3 of WHO Guidelines on the international packaging and shipping of vaccines.
Enacting Shipments
Air Shipments
Temperature sensitive shipments must be booked to the air company under the proper handling code and as “temperature-controlled health-care cargo”, as this is an exceptional service beyond that offered for general cargo.
Detailed information on managing air shipments of temperature-controlled cargo (at point of origin, by freight forwarder, by air carrier and ground handlers and at destination, etc.) is available in WHO Technical Report Series, No. 992, Annex 5, Supplement 12, Temperature-controlled transport operations by road and by air.
Road Shipments
For road shipments, it is critical to coordinate the delivery with the consignee before dispatch and confirm pick-up time and location.
To reduce as much as possible the time that vaccines are outside active devices and to exploit cold life of the passive container, prepare and pack the product in its designated packaging the same shipping day.
If using a third-party logistics provider, make sure that they are prequalified and approved for freight forwarding/transport.
If organising the shipment by own means, ensure that the designated vehicle is in good working condition and that the driver is aware of the cargo sensitiveness. Provide the driver with clear instructions and the necessary means to ensure proper load, handling and transport. This should include:
- Always placing the cold box in the vehicle in shadow and away from warm spots, avoiding the trunk of the vehicle as this is not a refrigerated space within the vehicle.
- The cold box should be secured firmly.
- Use of shaded and secure parking areas, minimising the time during which the vehicle is unattended.
- Avoid opening the cold chain containers during transit.
- Emergency contact information to call in case of breakdown or unexpected events.
Detailed information on managing road shipments of temperature-controlled cargo (including refrigerated road shipments) is available in WHO Technical Report Series, No. 992, Annex 5, Supplement 12, Temperature-controlled transport operations by road and by air.
Reception and Cold Chain Verification
The arrival of a vaccine shipment in a country, and its subsequent clearance through customs and transportation to the central vaccine store are the most critical stages in the shipping process. These are frequently the times when mistakes and delays occur, resulting in damage to the vaccines.
Reception at Customs
Regarding the customs clearance of the vaccines, the same procedures as described in the Customs topic apply, but with additional specific requirements linked to vaccine management. Note that requirements vary from country to country.
The first step in the customs clearance process, is contacting the following entities to obtain or verify the import procedures:
- National regulatory authorities (NRA) or head of customs in the destination country. To be cleared, the vaccines must have received marketing authorisation and a release certificate from the national regulatory authority.
- Local Ministry of Health (MOH): depending on country specific requirements, the MOH may issue a letter approving the shipment.
As reference, the general steps are:
- Submission of vaccine shipping documents (as soon as they are received) with a request to customs authority for the provisional clearance of shipment to the nominated Clearing and Forwarding (C&F) agent.
- C&F agent immediately processes the shipping documents as per established rules and regulations of government and contacts customs and airlines to coordinate the arrival, transport, checking and safe storage of the vaccines.
- Continuous contact is maintained well in advance with the concerned airlines to get accurate and updated information of the flight arrivals of the shipments.
- Once the flight arrives, immediate action is taken to release and take delivery of the vaccine shipment and to safely transport the vaccines to the cold storage locations.
- C&F agent checks the cold-chain monitor(s) and other mechanism (if necessary) to identify and reconfirm that the vaccines arrived in good condition before removing the shipment from the airport.
- Irrespective of the condition of the vaccines at the time of clearance, the C&F agent clears the vaccines and delivers as per regular procedures.
- The C&F agent informs the concerned official(s) in a timely manner and arranges for the cold room and the required staff to be ready and available to receive/store the vaccines.
- There should be a system in place to arrange to open the cold room and liaise/contact with the store keeper/cold room staff at any time (24-hours/day, including weekends and holidays).
- Under no circumstances can any vaccine be left unattended, or outside of the cold room in an open space.
- Unannounced shipments are cleared in time, like all other shipments.
- A reliable transport system including a refrigerated/insulated van should be made available at all times for effective transportation and delivery of the vaccines.
- In emergencies, the use of charter flights is very common. There are separate rules, regulations, systems and procedures for clearance of charter flights with vaccines including obtaining special permission for landing, fly over etc. and various no objection certificates (NOCs) from Ministry of Civil Aviation, etc.
Importing vaccines through ports that don’t have the adequate cold storage facility is not recommended. In the event of receiving a shipment of vaccines needing clearance in a port without cold storage facility or if the cold room is inaccessible, arrangements should be done for immediate release of the vaccine shipment. Coordination with the relevant authorities for an agile clearance and/or for safe and appropriate management and storage of vaccines at the airport are therefore needed.
Reception at the Storage Facility
Previous to reception make sure that a copy of the freight documents is available. Refer to the information about “Documentation” above.
Ensure priority unloading. Remove product from the vehicle and check that the number of boxes matches the number of boxes shown in the packing list. If it does not, note it conveniently in the provided waybill. Also indicate in the waybill if the shipping boxes were received in good condition and if all necessary labels on the outside of the shipping boxes were present.
Move the product immediately to the storage facility.
Open packaging, retrieve and inspect the temperature monitors, remove product from its passive shipping container and move it immediately to the correct temperature-controlled storage conditions.
If the temperature monitor (whichever is used: Cold Chain Monitor Card, Vaccine Vial Monitor, Electronic Temperature Indicators or Electronic Data Logging Monitors) shows a change that indicates potential damage to vaccines, take a picture, photocopy or scan that show alarm status. This information should be used to make decisions on whether to accept the product, or whether to quarantine it until an investigation has taken place and a final disposition has been made.
If using data loggers or tags that record time and temperature data that can be downloaded, retrieve and store conveniently time and temperature data. The point in time when a temperature excursion has occurred is important for the purchasing agency and the manufacturer so they can identify the cause of the excursion, take corrective measures, avoid similar situations in future shipments, and for insurance purposes.
Clearly identify vaccines in boxes in which the indicator shows exposure to temperatures that risk damage and keep them at the required temperature for further assessment of their condition. Do not discard vaccines until assessment is completed.
Verify that all necessary documents are present. Do not use the vaccines if the lot release certificate is missing. In that case, keep vaccines on hold in cold storage until the relevant document has been obtained from the vaccine manufacturer.
Report any relevant information to the carrier and to the appropriate personnel in your organisation. In case of loose or damage review insurance policy clauses and follow the insurance claim instructions.
Mass Vaccination Campaigns
Specific considerations must be taken regarding transport of vaccines and coolant packs when organising mass vaccination campaigns. In such events, numerous teams deploy simultaneously to different vaccination points carrying their own passive cold chain means. The number and rotation of coolant packs and the freezing capacity of the active cold chain must be carefully planned as the ice-pack turnover will be very high, and the period for their reconditioning can last more than 24 hours.
The number of icepacks and the freezing capacity of our cold chain, is calculated based on 3 variables: the number of vaccination teams simultaneously deployed, their expected daily vaccine consumption (doses/day), and the duration of the campaign. The expected daily consumption of vaccines for a vaccination team determines the required transport capacity for a team, and is based on its expected performance: the expected number of people to be vaccinated each day.
The performance of a vaccination team depends on the flow of people through the vaccination site, the number of working hours, and the number of vaccinators and vaccine preparers in each team. Though this will depend enormously on the environment (rural/urban), the organisation of the vaccination site, and the number of vaccine preparers per vaccinator, with a steady flow of people, one vaccinator can vaccinate on average 1 to 3 people per minute.
Based on the expected performance for a vaccination team, the number of cold boxes, vaccine carriers and icepacks per team is calculated. To calculate the number of icepacks, consider the cadence for its replacement: depending on the weather conditions and handling habits, icepacks in the cold box will need to be replaced daily or every 2 days, and extra icepacks for vaccine carriers may be needed throughout the day.
Next step is to define the quantity of freezers available for the vaccination campaign and determine if they have enough freezing capacity and storage capacity. The (water pack) freezing capacity is normally measured in kg of ice produced in 24h. And the freezer volume is normally provided in litres or in number of coolant packs of a certain volume that can be loaded in it. This information is part of the freezer technical specifications. Freezers powered by mains, depending on their volume and performance, may have a water-pack freezing capacity ranging from 7 kg to 40 kg per 24 hours. Standard SSD freezers may have considerably less water-pack freezing capacity: around 2 kg per 24 hours. If, for example, a particular freezer has storage capacity for 160 water-packs (0.6 L), and a water-pack freezing capacity of 7.2 kg per 24 hours, it will take approximately 13 days to freeze a full load of water-packs.
Taking into account the number of icepacks needed daily by the vaccination teams, and that the returned icepacks of the day won't be ready after a certain time (depending on the freezing capacity), a stock balance must be calculated and projected throughout the whole vaccination campaign. This will help to determine the minimum initial stock.
The figure below shows an example of the stock management of ice during a 15 days mass vaccination campaign with a total freezing capacity is 250 kg per 24 hours.
Temperature Monitoring
In order to maintain vaccine quality, it is essential to monitor the temperature of vaccines throughout the supply chain. Temperature monitoring devices are used to keep track of the temperature to which the vaccines and diluents are exposed. Based on the data from these devices, important decisions may be made.
Temperature monitoring devices and routines are used during every storage and during every transportation stage, until the vaccine is administered to the recipient. Depending on the supply chain level (central store, intermediary store or outreach and transportation container) different monitoring devices are used.
Monitoring Devices and Technologies
Devices for Cold Rooms and Freezer Rooms
For Cold Rooms and Freezer Rooms, monitoring devices integrate different functions like temperature and event loggers and alarm systems. They are fed by several sensors allowing the temperature monitoring in several room locations simultaneously. These systems are commonly configurable to suit user requirements by adjusting parameters such as logging interval or measurement unit.
Devices for Refrigerators and Freezers
The temperature in refrigerators and freezers is commonly monitored by the use of (analog) thermometers and (digital) data loggers. Data loggers are battery operated devices which measure and store data for a period of time. The time limit is the device memory. Some data logger models display the data instantly on an LCD screen while some other require data to be downloaded by USB or cable to a computer for later analysis.
One specific type of data logger is the 30-day electronic temperature recorder (30 DTR). This type of devices log the temperature at 10-minute intervals or less for 30 consecutive days on a rolling basis. They also record and display a 30-day history of any heat and freeze alarm violations that have occurred. Alarms are triggered if the temperature in the refrigerator drops to -0.5°C or below for 60 minutes or if it exceeds +8°C for a continuous period of 10 hours. As long as the temperature has remained within the recommended range, the device displays OK or a tick symbol. On some models, data can also be downloaded to a computer via USB interface. 30 DTRs are not designed to be used in vaccine freezers. Current models have built-in batteries with a battery alarm feature; the device must be discarded and replaced when the battery expires.
When monitoring the temperature in vaccine refrigerators (and vaccine freezers), it is recommended to apply a combined solution allowing spot temperature checks and alert displays. This can be easily achieved by the use of several devices. A convenient combination would be a 30 DTR (or other data logger) together with a stem thermometer acting as backup. When data loggers are not available, alternative alert devices such as electronic freeze indicators could be used. The use of a single standalone stem thermometer is not recommended as they only provide an instantaneous temperature reading. The minimum required monitoring equipment according to WHO recommendations is:
- Integrated digital thermometer.
- Stem thermometer for back-up.
- Electronic freeze indicator.
- VVMs where supplied.
Any device which requires frequent reading should be placed in an accessible location within the refrigerator unit and where they are unlikely to be damaged. Also consider the warmest and coolest places of the refrigerator model when placing the alert devices: If the refrigerator is used to store any freeze-sensitive vaccines, the device should preferably be placed in the coldest part of the refrigerator.
Devices for Transport Containers
For the temperature monitoring during transportation of vaccines, several monitoring devices are used:
Chemical Indicators - Also called markers or phase-change indicators). They are the most accessible and easy to use, they are based in a chemical impregnated onto a paperboard that changes its appearance under certain temperature. There are two types of chemical indicators:
- Threshold type.
- Progressive type.
Threshold Type chemical indicators provide a signal only when exposed to temperatures higher than (ascending indicator) or lower than (descending indicator) a predetermined threshold temperature. They are irreversible (thus, single-use) and are suitable for high or low temperatures.
Example of these devices are:
Progressive Type chemical indicators register multiple events in a cumulative way. Whenever the threshold temperature is exceeded the reaction is activated and the indicator starts to change. Further temperature violations increase the change process. The indicator for this type of device usually takes the form of a progressive colour change along a paper strip.
Cold Chain Monitor (CCM) Card
Paper-based temperature monitoring device which change colour irreversibly and at a constant rate. Indicator strips are attached to a card on which instructions for use are printed.
CCMs provide a warning when excessive heat exposure occurs during transport. They are used primarily to monitor the international shipment of freeze-dried vaccine consignments where dry ice is used. CCMs may also be appropriate for national vaccine shipments where the delivery takes several days.

Vaccine Vial Monitor (VVM):
Heat-sensitive label that gradually and irreversibly changes colour as the vaccine is exposed to heat. It warns the health worker when a vial should be discarded because the vaccine is likely to have been degraded by exposure to heat. For instructions on how to interpret VVM, refer to WHO How to Monitor Temperatures in the Vaccine Supply Chain.
Electronic Freeze Indicators - used to check if vaccines are exposed to freezing temperatures during storage or transport. The alarm indicator is triggered and displayed (changing from a “√” to an “X”) if exposed to temperatures lower than -0.5 °C for a continuous period of 60 minutes. To avoid malicious manipulation, once the alert is triggered, the alert is irreversible. If this happens the device is no longer usable and should be discarded. Otherwise, the device can be used until the built-in battery expires. The intermittent “dot” icon confirms active monitoring.
Electronic Shipping Indicators - more sophisticated devices that show if a product has been exposed to temperatures beyond the assigned alarm settings. They record the temperature at regular intervals during a certain period (normally not exceeding 20 days due to memory overflow). They have a digital display that reflects if the vaccine being shipped crossed the alarm thresholds.
Shipping indicators are mounted on a coloured card (yellow or blue) with a data entry section on one side, which the manufacturer fills in at the point of dispatch, and an instruction and interpretation section on the reverse side for the recipient. Yellow indicators are for freeze-sensitive vaccines, and blue indicators are for heat-sensitive vaccines.
These devices are not re-usable once alarm conditions are triggered or the programmed time elapses. In addition, the heat and/or freeze alarm thresholds are product-specific, which means that the device is not reusable with different vaccines. Some brands are able to download the temperature data to a computer. This enables recipients to determine whether shipments have been exposed to excessively high or low temperatures; it also helps the procurement agency to determine when, where and to what extent temperature limits have been exceeded.
Temperature Monitoring Routines
Temperature-monitoring and record-keeping are required to make sure that each vial of vaccine is maintained under appropriate conditions. The data gathered from temperature monitoring devices must be recorded and analysed on a regular basis to demonstrate that vaccines are being stored and transported at the correct temperatures.
A standard recommended practice for secondary/intermediary storage facilities and health facilities is to check and record the temperatures in the refrigerators at least twice a day, in separated intervals: first thing in the morning and at the end of the working day is considered a good practice. Alert displays should also be revised. This should be done every day of the year, including days off.
At each spot check the temperature should be recorded. This should be done on a standard temperature chart. The chart should be attached to the door of the specific fridge/refrigerator being monitored. Completed charts should be filed together for future reference.
Source: https://watch.immunizationacademy.com/en
For monitoring the temperature of cold rooms, temperature checks should be also done twice daily, but at each inspection, it should be read the temperature record for the entire period since the last reading.
If during a temperature check in a cold room or refrigerator:
- Temperature is at or below 0°C or a freeze alert is activated: immediate action should be taken to correct the low temperature and to ensure that the problem does not arise again. The agreed contingency plan should be triggered and a report completed.
- Temperature is between +8°C and +10°C: check that the refrigeration unit is working, monitor the situation closely in the following hours and take appropriate action if the temperature is not within the normal range at the time of the next inspection. If there was a temporary power failure, no further action on fridge or cold room would be necessary.
- Temperature is above +10°C: activate immediately the agreed contingency plan, and make a report.
If during a temperature check in a freezer room or chest freezer:
- Temperature is below -25°C: Adjust thermostat. Check that the temperature is within the normal range at the time of the next inspection.
- Temperature is above -15°C: Check that the refrigeration unit is working, monitor the situation closely and take appropriate action if conditions are not normal at the time of the next inspection. If there has been a temporary power failure, no further action is necessary. A temporary rise to +10°C is permissible following a temporary power cut.
- Temperature is above +10°C: Take immediate action to implement the agreed contingency plan, and make a report.
Managing Cold Chain Ruptures
Every storage facility should develop a contingency plan for the event of a cold chain rupture. The plan should be accessible, allowing the staff to know what to do when the event happens. Contact numbers for key persons to be urgently contacted in such event and emergency cold chain storage capacity for back-up is information that must be included.
Passive cold chain can be used as temporary backup storage. In that case:
- Provide enough cold boxes to store products temporarily.
- Always have the required number of frozen icepacks available for the cold boxes.
- Increase the frequency to monitor the temperature.
In case of cold chain rupture, the following actions should be taken:
- Identify the affected products, mark them and place them in quarantine to avoid its use temporarily. A quarantine area should be clearly marked for this purpose in one functioning cold chain equipment.
- Write a cold chain breakdown report and send it to the relevant persons. It must be submitted as soon as possible. The report should include information about the products (name, manufacturer, batch number, expire date, quantity) and the details of the Cold chain rupture (temperature range, exposure time, source of the alert).
- Wait for pharmacist recommendations. Even if some cold chain break occurred, the vaccine could be used under certain instructions. In case that the vaccine must be discarded, proceed according to the national regulation.
Cold Chain Tools and Resources
Templates and Tools
Sites and Resources
- Technical Network for Strengthening Immunization Services
- UNICEF Cold Chain Technical Support
- WHO Performance, Quality and Safety (PQS)
- Cold Chain Evaluation, MSF
- Decommissioning and Safe Disposal of Cold Chain Equipment, 2018, UNICEF-WHO
- Delivering Vaccines: A Cost Comparison of In-Country Vaccine Transport Container Options, 2013, PATH-WHO
- Effective Vaccine Store Management Initiative, 2005, WHO-UNICEF
- Effective Vaccine Management (EVM) model standard operating procedures, 2013, WHO
- Guideline For Establishing Or Improving Primary And Intermediate Vaccine Stores, 2002, WHO
- Guidelines on the international packaging and shipping of vaccines, 2019, WHO
- How to calculate vaccine volumes and cold chain capacity requirements, 2017, WHO/IVB
- How to Monitor Temperatures in the Vaccine Supply Chain, 2015, WHO-IVB
- How to use passive containers and coolant-packs, 2015, WHO-IVB
- Immunization in practice: A practical guide for health staff. Geneva, 2015, WHO
- Introducing Solar-powered Vaccine refrigerator and freezer systems, A guide for managers in national immunization programmes, 2015, WHO
- Procurement Guidelines for Walk-In Cold Rooms And Freezer Rooms, 2020, UNICEF
- Procurement Guidelines, Compression System Refrigerators and Freezers, 2014, UNICEF
- Procurement Guidelines, Solar Direct Drive Refrigerators and Freezers, 2014, UNICEF
- Procurement Guidelines, Temperature Monitoring Devices, 2016, UNICEF
- Procurement Guidelines, Vaccine Carriers and Cold Boxes, 2016, UNICEF
- Study protocol for temperature monitoring in the vaccine cold chain, 2011, WHO-IVB
- Technical Report Series, No. 992, Annex 5, Supplement 12, Temperature-controlled transport operations by road and by air, 2015, WHO
- Thermostability of vaccines, 1998, WHO/GPV/98.07
- Total Cost of Ownership Tool for Cold Chain Equipment, 2019, PATH
- User’s handbook for vaccine cold rooms and freezer rooms, 2002, WHO