Cold Chain Storage for Vaccines
Estimating the Required Storage
The required storage capacity will determine the right cold chain solution. The required storage capacity for a given cold chain will depend on:
- The quantity
- The type of vaccines to be stored
Quantity of Vaccines - will depend on the targeted population to be immunised, the vaccine consumption and the vaccine supply frequency and reliability.
The calculation of vaccine consumption requires a careful analysis of existing public health data, targeted coverage and a consideration of future requirements. It is important to plan not only for current needs, but also for future needs.
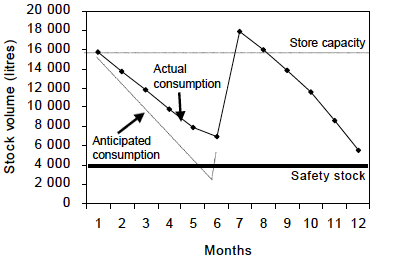
It is advised to add a safety margin in the storage capacity to respond to stock peaks that may overwhelm the cold chain storage capacity (see figure below, taken from WHO Guideline for establishing or improving primary and intermediate vaccine stores). Stock peaks occur when the volume of vaccine actually distributed in the period between any two supply intervals is less than the volume predicted to be distributed during this period. They can also arise if a vaccine delivery arrives earlier than anticipated. Overstocking and under-stocking are also caused by seasonal fluctuations in demand, campaigns, National Immunisation Days and so forth.
In addition to stock peaks, it is recommended to maintain some extra store capacity as back-up to overcome equipment malfunctioning or maintenance (i.e., emptying a refrigerator for defrosting).
Type of Vaccine - of key importance because different vaccines have different presentations. The most common are vials (or ampules), but also, single-dose pre-filled syringes may be stored. Depending on the vaccine, vials can contain different number of doses, normally 1, 10 or 20 doses. Because of these different presentations, the key variables used to calculate the required volume for vaccine storage are the number of doses to be stored and the estimated volume per dose. The estimated volume per dose (or packed vaccine volume) quantifies the space needed to store or transport vaccines and diluents and will depend on the number of doses per vial, the physical size of the vial or ampule (primary package) and the bulkiness of the external packaging (secondary packages).
It is important to consider that some presentations include the diluent in the same packaging as the vaccine. In such cases it is necessary to refrigerate the diluent as well as the vaccine. In all cases, diluents should be refrigerated 24h prior to vaccine preparation. Refrigeration of diluents is normally done in the last step of the vaccine supply chain.
Whenever possible, the packed vaccine volume per dose should be calculated using data from the vaccine manufacturer or supplier. The World Health Organisation (WHO) and the United Nations Children’s Fund (UNICEF) online databases provide access to data on the packed vaccine volume per dose or the dimensions of vaccine and diluent packaging for WHO-pre-qualified vaccines.
To calculate required vaccine storage capacity, it is recommended to use the WHO guidance document for vaccine volume calculation: How to calculate vaccine volumes and cold chain capacity requirements.
Measuring the length, width and height of the vaccine packaging and dividing the resulting volume by the total number of vaccine doses contained in that packaging, may be an alternative method for vaccine volume calculation.