A specific component to health supply chains that is frequently overlooked or underestimated by humanitarian organisations is the overall regulatory framework in which management of health commodities resides. Different operating contexts will have extremely different regulations and laws governing the procurement, storage, transportation and distribution of pharmaceuticals and other health items. In many contexts special certifications or permits are required to even handle health items, and in some cases humanitarian agencies may outright be incapable of managing their own health supplies without utilising an accredited third party.
Over the past few decades there has been increasing attention to how health items are managed on both a national and international level, and many traditional humanitarian emergencies may now be facing stricter regulations than before. Alternately, some humanitarian contexts have virtually no local or national regulations pertaining to the management of health items, and responding organisations must do their best to maintain a minimum level of quality for the management of health items.
Humanitarian organisations should be aware of local regulations when they begin a health-related project in any given country and should consult with national or local Ministries of Health, Food and Drugs Administration Authorities and National Drug Regulatory Authorities, or other relevant ministries about the prevailing laws and regulations prior to beginning activities.
Good Distribution Practice (GDP) for Medical Products
Good Distribution Practice (GDP) is a set of standards for all supply chain actors involved to work with a common objective of ensuring product quality safety and efficacy when delivered to patients. GDP applies equally to forward supply, to reverse logistics, to commercial supply chains, to private and public health supply chains, whether items are procured directly or donated. The objective of adherence to GDP is to ensure that goods are supplied from the manufacturer to the population with minimal impact on their quality, safety and efficacy, and to ensure the avoidance of infiltration of falsified, counterfeit or substandard products into legitimate supply chains. GDP is the responsibility of all actors’ participants in the distribution process to ensure that procedures are designed to protect the products and the recipient population.
GDP encompasses many aspects of the management of pharmaceuticals and health commodities that humanitarian organisations might encounter, however there are many other categories of quality assurance management for categories for health supply chains, including:
- Good Manufacturing Practice (GMP)
- Good Pharmaceutical Practice (GPP)
- Good Storage Practices (GSP)
- Good Trade and Distribution Practice (GTDP)
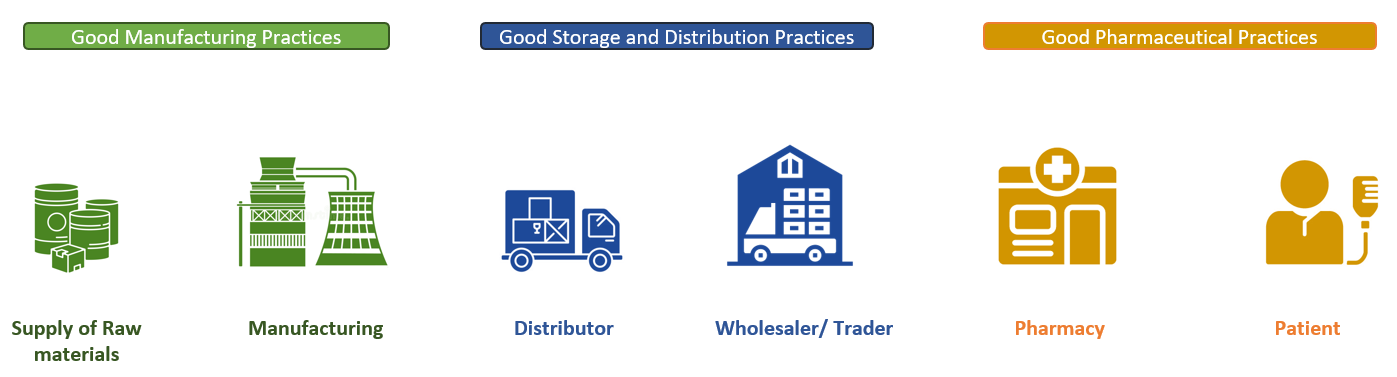
Often, the different special categories of practice are all labelled as GDP. The specific nomenclature is not as important to humanitarian actors – the important part is that humanitarian organisations managing a supply chain of health items understand what their obligations are, based both on the type of commodities and the prevailing regulations in the context of operation. The point of a GDP is to ensure that the following components of a health supply chain are adequately planned and developed:
- Traceability and Inventory Management.
- Necessary Equipment.
- Storage and Transport Standards.
- Documented procedures.
- Responsibilities for GDP set out in job descriptions.
- Quality risk management.
- Management of Outsourcing.
- Management of Change, Deviations and Corrective Actions and Preventive Action (CAPA).
- Self-inspections.
- Systems for handling returns, complaints and recalls.
- Notification to senior management of GDP compliance and performance.
- Training of personnel.
The World Health Organisation (WHO) maintains detailed guidance on GPD that is regularly updated, and is available to all healthcare practitioners. However, many countries and national authorities maintain their own specific GDP requirements that vary from context to context and require their own study and compliance. Many Ministries of Health (MoH) produce publications or maintain websites with regulations and resources available for the public - Humanitarian response organisations should inquire about GDP regulations in any context in which they operate prior to enacting procurement or establishing health activities.

