Humanitarian logistics personnel may be required to organise the transportation of clinical samples from the outbreak location to a reference laboratory may be required, especially during disease outbreaks, such Ebola Haemorrhagic Fever. The transportation of samples is usually handled by either by the local government, or by a WHO representative, or by a specialised agency tasked with the process in the local context.
Clinical and biological samples are considered "dangerous goods", and transport of these is subject to very strict regulations. Before transporting clinical samples always consult local regulations and international best practice. Commercial air and sea transporters will often have clear guidelines on the transportation of clinical and biological samples – reference the dangerous good section of this guide for more information. In absence of a clear local regulation, humanitarian agencies might refer to WHO’s “Guidance on regulations for the transport of Infectious Substances”.
Biologic samples are separated into two different categories when prepared for shipping:
| Category A | An infectious substance which is transported in a form that, when exposure to it occurs, is capable of causing permanent disability, life-threatening or fatal disease in otherwise healthy humans or animals. If a Category A substance were released from the craft carrying it and/or protective packaging used during the transportation, it could have severe consequences on the health of any humans or animals that came in contact with it. |
|---|---|
| Category B | Infectious substances that contain biological agents, capable of causing infection in humans or animals, but NOT meeting the criteria for Category A (i.e. the consequences of an infection are not considered severely disabling or life-threatening).
|
Adapted from: WHO’s - Guidance on regulations for the transport of Infectious Substances
Packaging
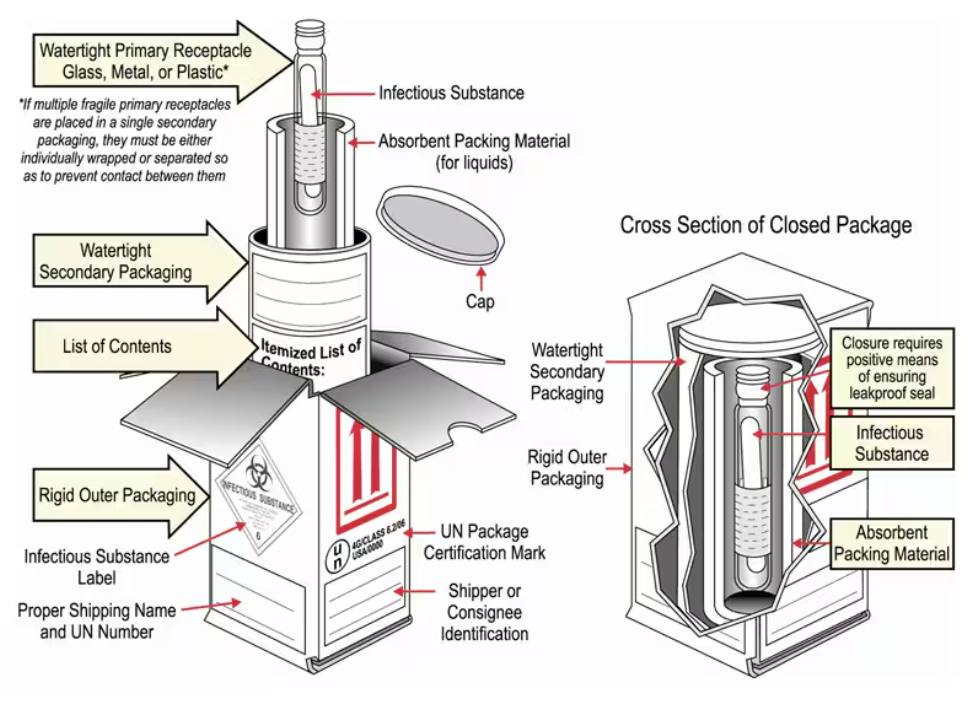
Both Category A and Category B substances have their own forms of approved packaging, and all samples must be transported in their respective approved packaging, usually some form of triple packing. Consider that in some contexts, this type of packaging won’t be available to be purchased locally. Certain health actors or specialised medical agencies may have stock available.
The system for transporting samples consists of three layers:
- Primary container containing the sample: Tube or bottle tightly closed and labelled.
- Secondary container intended to protect the primary container: Waterproof box/tube (Category A) or plastic bag (Category B) with enough absorbent material to absorb all the liquid in case of breakage.
- Outer packaging intended to protect the secondary container: Reinforced cardboard box with UN labelling.
Example Packaging for “Category A” Biological Samples
Example Packaging for “Category B” Biological Samples
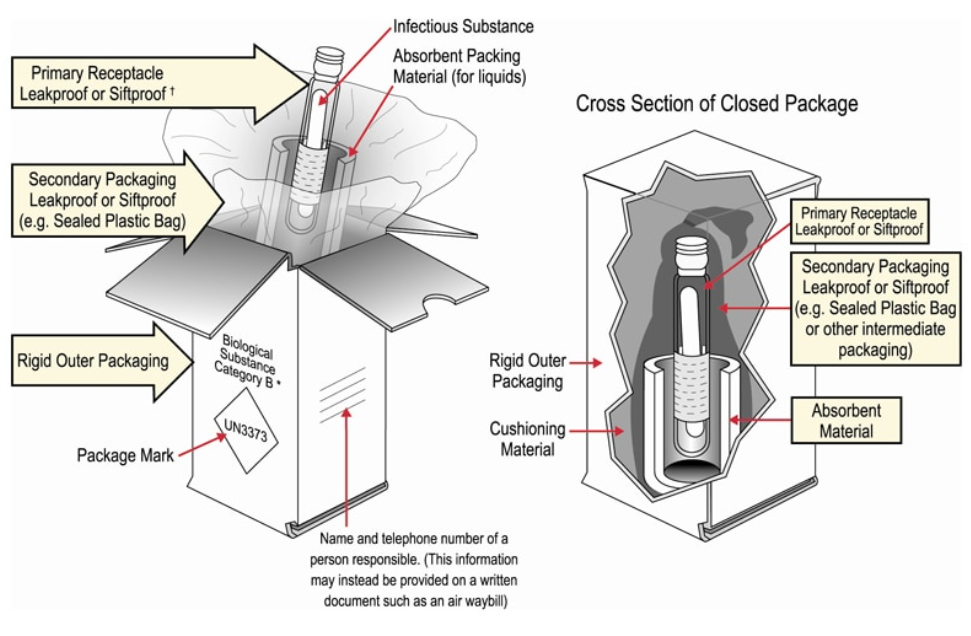
The choice of container depends on the classification of the sample to be transported and whether or not it is necessary to transport the sample at controlled temperature; some samples will require +2°C to +8°C temperature.
Usually, individual transported biological samples will be uniquely identified with information such as the name or patient code number and date/place of collection and will be accompanied by relevant clinical and epidemiological information. Information to be contained on the on the outer packaging of the box should include:
- Shipper.
- Consignee.
- Emergency contact: mention the name and the phone number of the person to contact in case of emergency (i.e., incidental opening or leakage).
- UN approved marking and product category/class.
- Net capacity of sample only.
- Mandatory marking: "Infectious substance" logo and additional required approval markings.
The shipper is responsible for classifying, declaring, packaging and labelling the samples. Any transporter or service provider involved in the transportation chain, must be informed about the material being sent. If there is any problem during the transport, the shipper must be able to prove that he has strictly followed the regulations. If humanitarian organisations organising the transport of biological samples have any questions on labelling, they may also consult their freight forwarder or transport company.
The person enacting the shipment be sure to inform the receiving party in advance, specifying the nature of the sample as well as the planned shipping date to ensure readiness to receive the sample. In some cases, biological samples will be delivered to third party laboratories or government offices who may have very little understanding of the humanitarian operation. Shippers should also tell transport companies well in advance as well, as they may have their own protocols for handling and managing these types of shipments.
Below is a list of UN ID numbers and packing instruction per category that should be included with every shipment.
| UN No. | Proper Shipping Name | Category | Hazard Class | Packing Instructions |
|---|---|---|---|---|
| UN2814 | Infectious substance affecting humans | Category A | 6.2 | 620 |
| UN2900 | Infectious substances affecting animals | Category A | 6.2 | 620 |
| UN3549 | Medical waste, Category A, affecting animals only, solid | Category A | 6.2 | 622 |
| UN3549 | Medical waste, Category A, affecting humans, solid. | Category A | 6.2 | 622 |
| UN3291 | Biomedical waste, n.o.s., Clinical waste, unspecified, n.o.s. or medical waste, n.o.s. or regulated medical waste, n.o.s. | Category B | 6.2 | 621 |
| UN3373 | Biomedical Substance Category B | Category B | 6.2 | 650 |
More information on identifying dangerous goods categories can be found in the dangerous goods section of this guide.

